Local Researchers Participate In Testing Of New FDA-Approved Alzheimer's Drug
Hope is being restored for those with loved ones fighting Alzheimer's. The Food and Drug Administration approved the first drug that can slow the disease.Thursday, June 17th 2021, 10:08 am
OKLAHOMA CITY -
Researchers’ goal is to cure Alzheimer's, but scientists are doing so one step at a time.
A patient in Rhode Island received the first infusion Wednesday of a newly-approved drug to treat the disease but some took issue with that approval.
“I know there is controversy about this approval,” Butler Hospital director of neurology Dr. Stephen Salloway said. “I think the FDA took a very forward-thinking position to make this treatment available, to have access for patients under accelerated approval.”
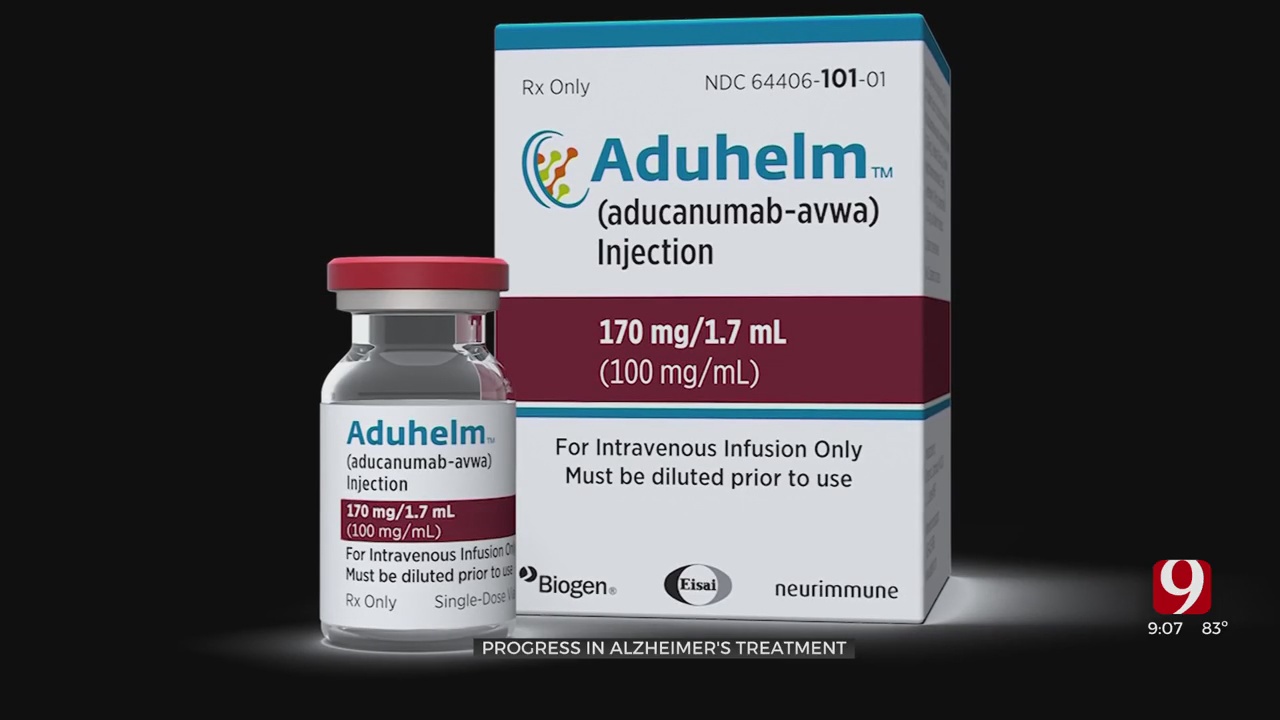
The controversy surrounds a drug called Aduhelm. Critics said it's can be a big hit to patients' pocketbooks. Studies also showed conflicting results, but scientists said the drug is a huge step forward.
Aduhelm was tested at the Lynn Institute here in Oklahoma.
Researchers said the drug requires a monthly infusion that slows cognitive decline in Alzheimer's patients experiencing mild symptoms.
“It's not a magic pill. It won't cure Alzheimer's and likely will not revert anything lost," Lynn Institute CEO Carlos Blanco said. "It will slow down the progression of the disease."
Blanco said the drug will build momentum against the disease as more medicine progresses with more medications.
Even now, a new drug from Eli Lilly is in trials, which has Lynn Institute researchers excited.
"It's the same type of mechanism,” Blanco said. “In the early trials, it appears to show additional benefits.”
The Lynn Institute said you can encourage loved ones to participate in a trial.
More Like This
April 13th, 2024
Top Headlines
May 1st, 2024
May 1st, 2024
May 1st, 2024